箴言
在科学上没有平坦的大道,只有那些不畏艰险沿着陡峭山路攀登的人,才有希望达到光辉的顶点。
----马克思
-----------------------------------------------
合作研究
请有兴趣的研究组联系我们。欢迎任何形式的合作,尤其是在自组装、水凝胶以及生物医药等方向的合作。
------------------------------------------
研究成果
Fang, J.; Li, T.; Lee, J.; Im, D.; Xu, L.; Liu, Y.; Seo, J.; Zhang, W.-B.* A single-domain protein catenane of dihydrofolate reductase. Natl. Sci. Rev. 2023, 10, nwad304. https://doi.org/10.1093/nsr/nwad304
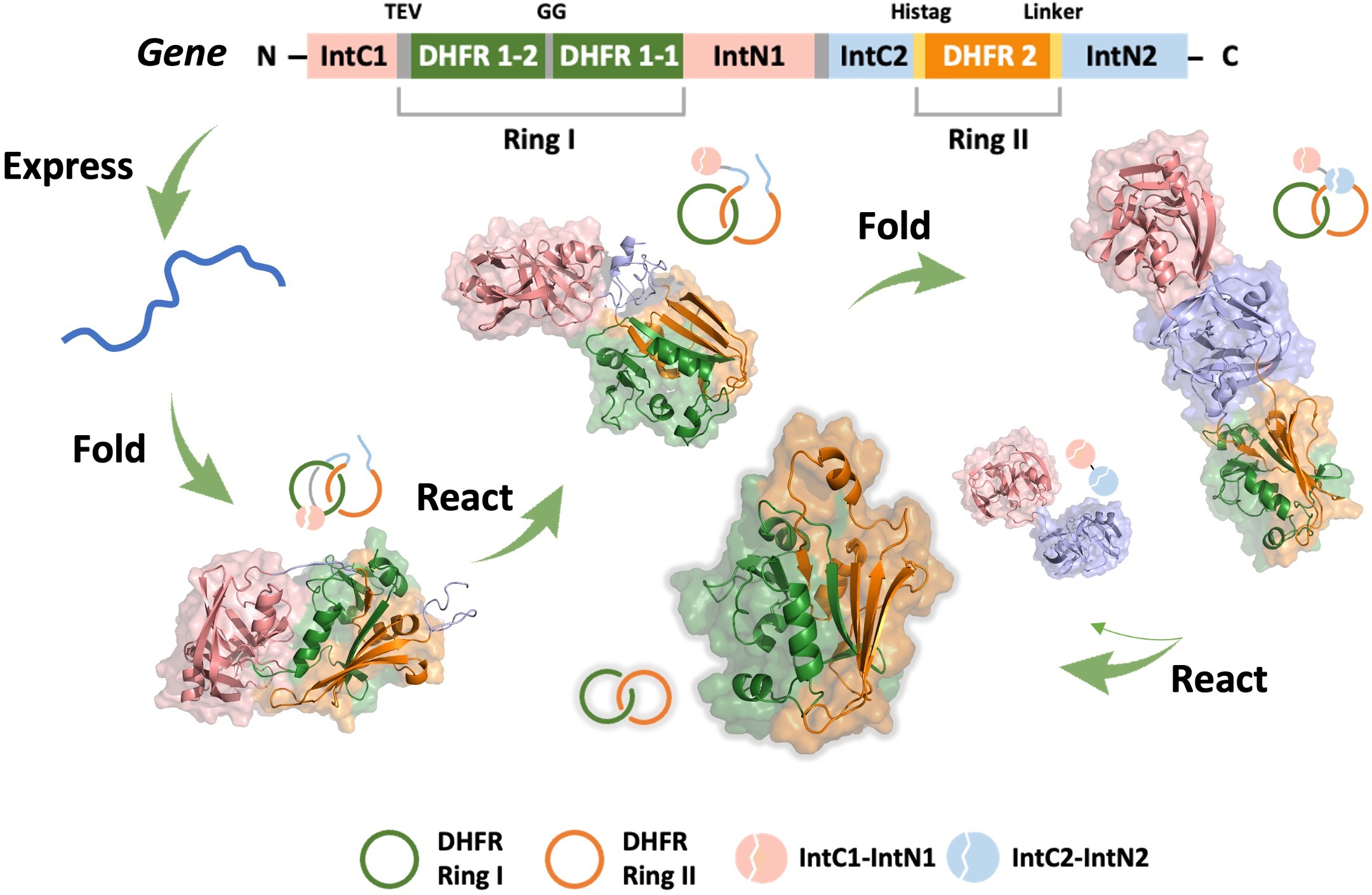
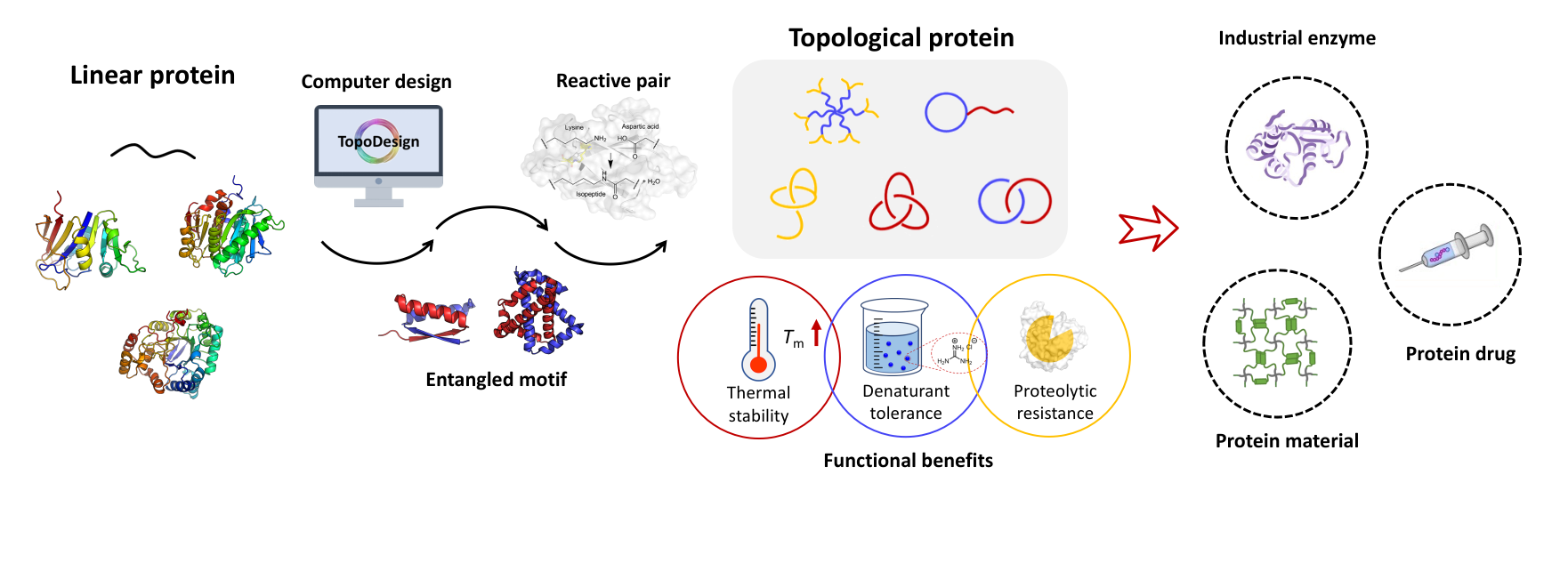
A single-domain protein catenane refers to two mechanically interlocked polypeptide rings that fold synergistically into a compact and integrated structure, which is extremely rare in nature. Herein, we report a single-domain protein catenane of dihydrofolate reductase (cat-DHFR). The design was achieved by rewiring the connectivity between secondary motifs to introduce artificial entanglement and the synthesis was readily accomplished by a series of programmed streamlined post-translational processing events in cells without any additional in vitro reactions. The target molecule contains few exogenous motifs and has been thoroughly characterized by combined techniques of LC-MS, SDS-PAGE, protease cleavage experiment, and ion mobility mass spectrometry. Compared to the linear control, cat-DHFR retains the catalytic capability and exhibits enhanced stability against thermal or chemical denaturation due to conformational restriction. The results suggest that linear proteins may be converted into concatenated single-domain counterparts with almost identical chemical composition, well-preserved function, and elevated stability, which represents an entirely new horizon in protein science.