最新热点
联系我们
张文彬课题组
地址:北京市海淀区成府路202号
伟德国际1946官网
邮编:100871
电话:010-62766876
电邮:wenbin@pku.edu.cn

请扫以上二维码关注我们课题组的公众号。
我们将定期推送组会每周精读和泛读文献介绍以及课题组的最近新闻!
--------------------------------------------
新闻中心
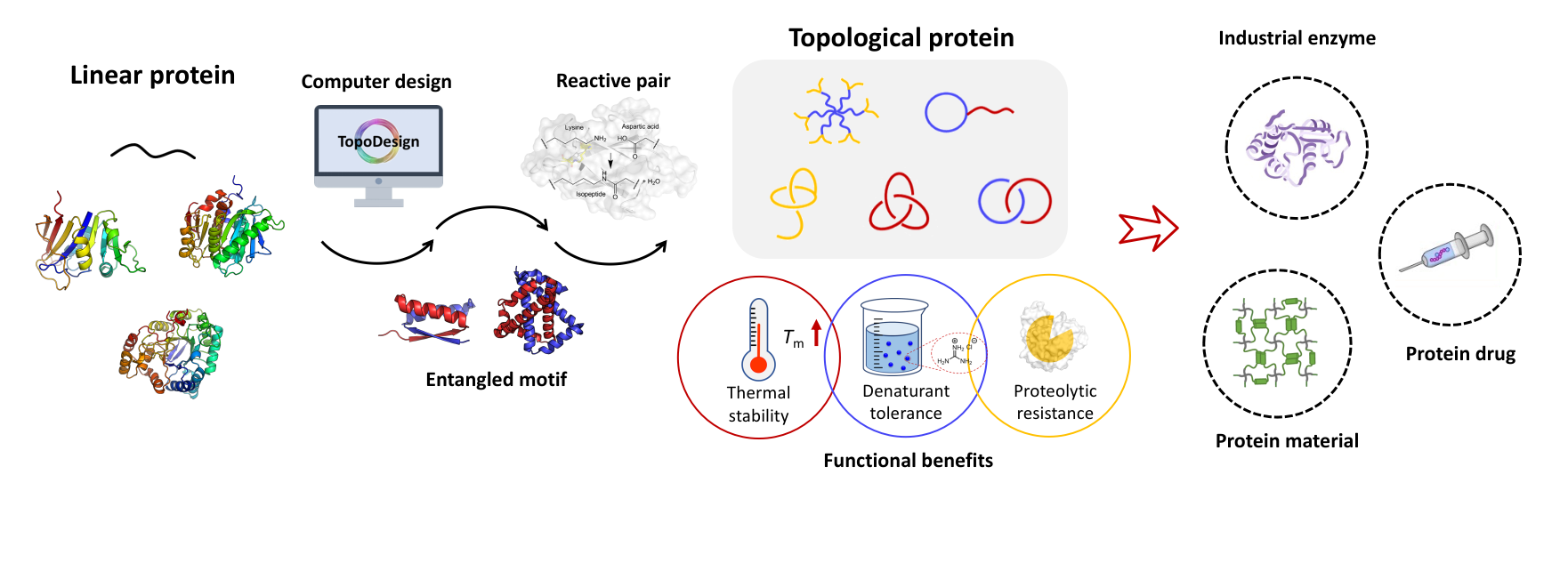
Proteins are the workhorses of nature. With precise and hierarchical structures, they possess unique functions. Some are the major components of the extracellular matrices (ECM), supporting cell growth as long as tissue generation. Inspired by them, we seek for a strategy to construct protein materials, such as hydrogels, to combat diseases and better understand life. We utilize a special tool of genetically encoded protein reaction pairs with high specificity and high efficiency. Combining protein engineering with polymer chemistry, we hope to open an avenue toward next generation biomaterials that are readily prepared, yet highly smart and bioactive.1
Exemplary projects are discussed below:
I. The Network of Spies
An all-protein-based hydrogel, a.k.a. the Network of Spies, are formed by the isopeptide crosslinking between the SpyCatcher-SpyTag reactive pairs. This pair was engineered and optimized from, by Howarth group, the CnaB2 domain in the pili of Streptococcus pyogenes that forms intramolecular isopeptide bonds. These genetically encoded reactive pairs have been successfully applied to expand the topology of functional proteins and to drive protein gelation. We believe that this pair is a powerful tool to create soft protein hydrogels totally compatible with cells and provides an easy platform for engineering other properties.

II. Protein Catenanes
Catenanes are molecules having two mechanically interlocked rings, which have already been designed and synthesized in the past decades for small-molecules. However, there were very few reports on protein catenanes. The only artificial protein catenane is derived from the intertwined domain in tumor suppressor p53 (p53 dim, short as X) locked by native chemical ligation. Such catenane structure gives higher thermos-stability and resistance to denaturants and proteases. Inspired by this, we developed an in situ catenation method by using the SpyCatcher-SpyTag reactive pairs that allows the direct cellular synthesis of protein catenanes. This improvement allows various proteins of interest (POI) to intertwine with each other and ligate with itself. By now we have been able to express concatenated intrinsically disordered proteins or functional proteins in E.coli. We are pushing the technology forward to test property improvement of concatenated protein molecules.