箴言
在科学上没有平坦的大道,只有那些不畏艰险沿着陡峭山路攀登的人,才有希望达到光辉的顶点。
----马克思
-----------------------------------------------
合作研究
请有兴趣的研究组联系我们。欢迎任何形式的合作,尤其是在自组装、水凝胶以及生物医药等方向的合作。
------------------------------------------
研究成果
Zhang, F.; Liu, Y.; Shao, Y.; Zhang, W.-B.* Active Template Synthesis of Protein [n]Catenanes Using Engineered Peptide–Peptide Ligation Tools. CCS Chem. 2023, DOI: 10.31635/ccschem.023.202302762. https://doi.org/10.31635/ccschem.023.202302762
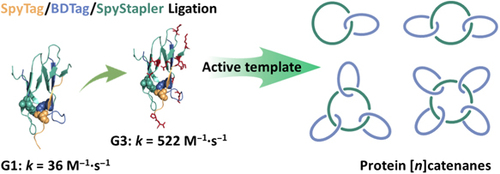
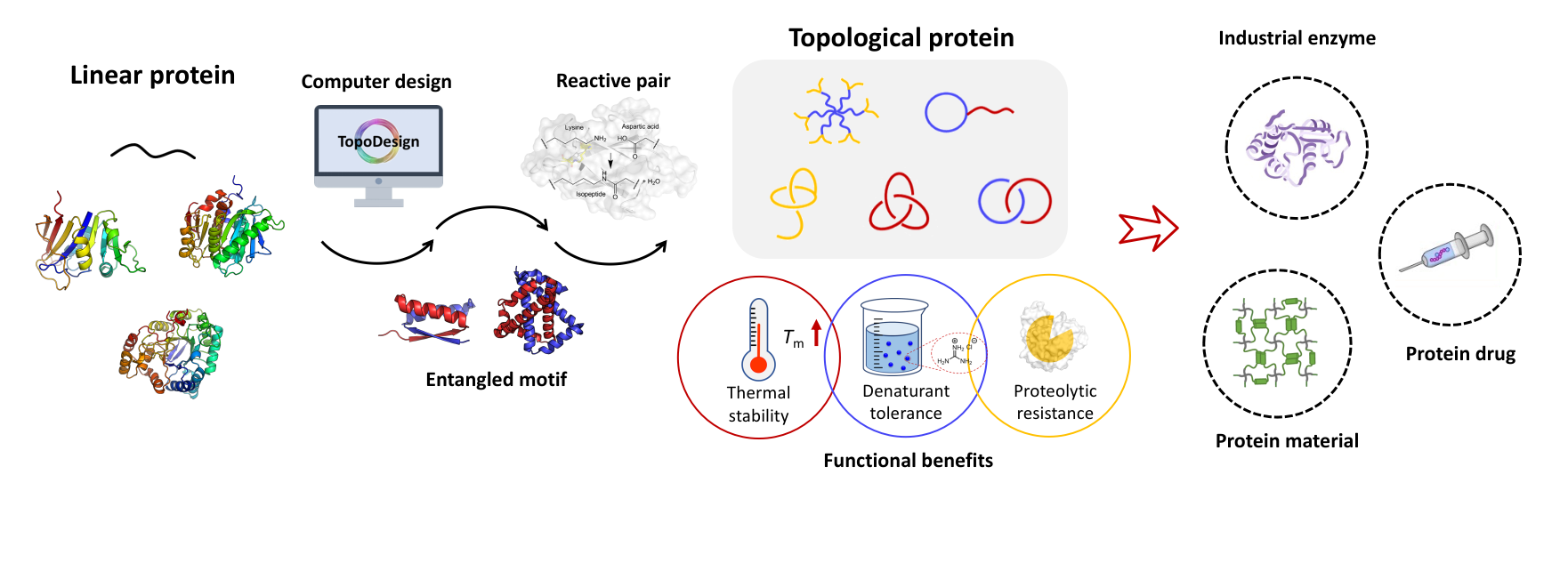
The expansion of protein topological diversity requires new and efficient synthetic tools. Herein, we report the second and third generations of the SpyStapler-mediated SpyTag/BDTag ligation system for the efficient synthesis of 4-arm star proteins and the repurposing of the third generation as an active template to enable the synthesis of higher-order protein [n]catenanes (n = 3, 4, and 5). SpyStapler003 has a higher affinity to its cognate SpyTag and BDTag reactive pairs relative to the original SpyStapler. Hence, it can overcome much more profound steric hindrance in protein ligation and improve the efficiency of the resulting active template tool to facilitate the construction of radial protein [n]catenanes. Various proteins of interest, such as dihydrofolate reductase and the nanobody KN035, can be modularly incorporated into the [n]catenanes with intact activity. Combination of passive and active template strategies gives rise to linear protein [4]catenanes, which further expands the current topological diversity. Moreover, higher-order protein catenation not only leads to enhanced thermal stability and proteolytic resistance but also higher affinity of the nanobody via multivalent effects. Our study provides tools useful for bioconjugation and new topological protein scaffolds for the multivalent display of enzymes and antibodies.