箴言
在科学上没有平坦的大道,只有那些不畏艰险沿着陡峭山路攀登的人,才有希望达到光辉的顶点。
----马克思
-----------------------------------------------
合作研究
请有兴趣的研究组联系我们。欢迎任何形式的合作,尤其是在自组装、水凝胶以及生物医药等方向的合作。
------------------------------------------
研究成果
132.Wu, W.-H.; Bai, X.; Shao, Y.; Yang, C.; Wei, J.; Wei, W.;* Zhang, W.-B.* Higher Order Protein Catenation Leads to Artificial Antibody with Enhanced Affinity and in Vivo Stability. J. Am. Chem. Soc. 2021, 143, 18029-18040.
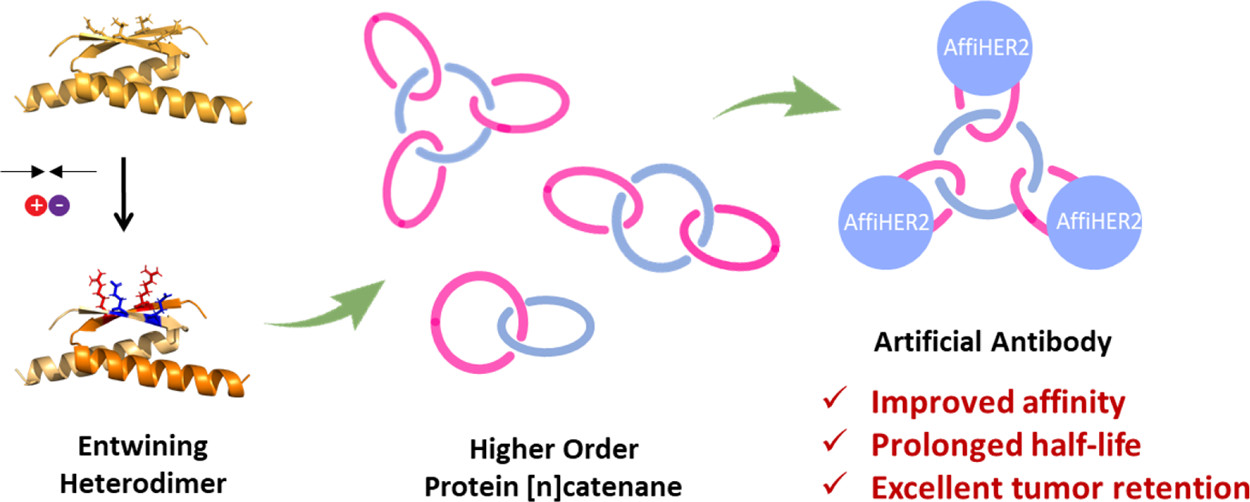
The chemical topology is a unique dimension for protein engineering, yet the topological diversity and architectural complexity of proteins remain largely untapped. Herein, we report the biosynthesis of complex topological proteins using a rationally engineered, cross-entwining peptide heterodimer motif derived from p53dim (an entangled homodimeric mutant of the tetramerization domain of the tumor suppressor protein p53). The incorporation of an electrostatic interaction at specific sites converts the p53dim homodimer motif into a pair of heterodimer motifs with high specificity for directing chain entanglement upon folding. Its combination with split-intein-mediated ligation and/or SpyTag/SpyCatcher chemistry facilitates the programmed synthesis of protein heterocatenane or [n]catenanes in cells, leading to a general and modular approach to complex protein catenanes containing various proteins of interest. Concatenation enhances not only the target protein’s affinity but also the in vivo stability as shown by its prolonged circulation time in blood. As a proof of concept, artificial antibodies have been developed by embedding a human epidermal growth factor receptor 2-specific affibody onto the [n]catenane scaffolds and shown to exhibit a higher affinity and a better pharmacokinetic profile than the wild-type affibody. These results suggest that topology engineering holds great promise in the development of therapeutic proteins.