箴言
在科学上没有平坦的大道,只有那些不畏艰险沿着陡峭山路攀登的人,才有希望达到光辉的顶点。
----马克思
-----------------------------------------------
合作研究
请有兴趣的研究组联系我们。欢迎任何形式的合作,尤其是在自组装、水凝胶以及生物医药等方向的合作。
------------------------------------------
研究成果
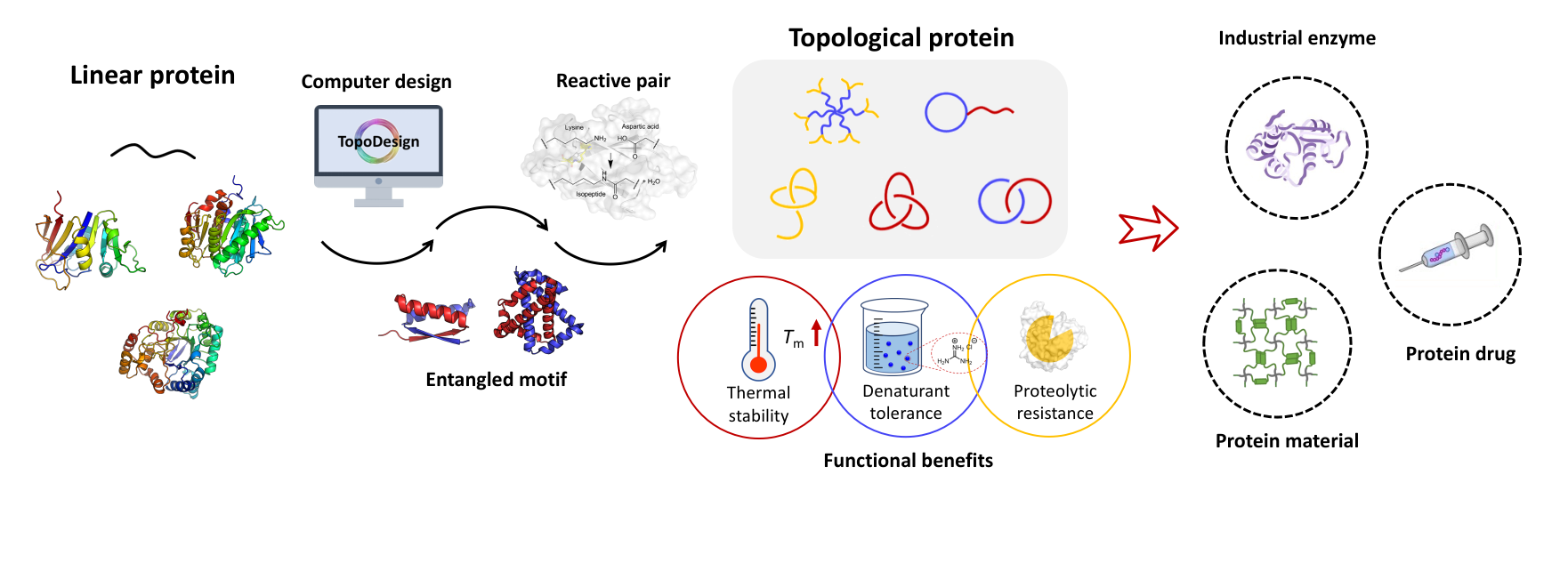
Zhang, W.-B.;† Sun, F.;† Tirrell, D. A.;* Arnold, F. H.* Controlling Macromolecular Topology with Genetically Encoded SpyTag-SpyCatcher Chemistry. J. Am. Chem. Soc., 2013, 135, 13988-97. [Link] [PDF]

Abstract
Control of molecular topology constitutes a fundamental challenge in macromolecular chemistry. Here we describe the synthesis and characterization of artificial elastin-like proteins (ELPs) with unconventional nonlinear topologies including circular, tadpole, star, and H-shaped proteins using genetically encoded SpyTag–SpyCatcher chemistry. SpyTag is a short polypeptide that binds its protein partner SpyCatcher and forms isopeptide bonds under physiological conditions. Sequences encoding SpyTag and SpyCatcher can be strategically placed into ELP genes to direct post-translational topological modification in situ. Placement of SpyTag at the N-terminus and SpyCatcher at the C-terminus directs formation of circular ELPs. Induction of expression at 16 °C with 10 μM IPTG yields 80% monomeric cyclic protein. When SpyTag is placed in the middle of the chain, it exhibits an even stronger tendency toward cyclization, yielding up to 94% monomeric tadpole proteins. Telechelic ELPs containing either SpyTag or SpyCatcher can be expressed, purified, and then coupled spontaneously upon mixing in vitro. Block proteins, 3-arm or 4-arm star proteins, and H-shaped proteins have been prepared, with the folded CnaB2 domain that results from the SpyTag–SpyCatcher reaction as the molecular core or branch junction. The modular character of the SpyTag–SpyCatcher strategy should make it useful for preparing nonlinear macromolecules of diverse sequence and structure.