Synthesis of Pyrimidines from Dinitrogen and Carbon
Xianghui Shi#, Qianru Wang#, Chao Qin#, Li-Jun Wu, Yuanjin Chen, Gao-Xiang Wang, Yongli Cai, Wenbo Gao, Teng He, Junnian Wei*, Jianping Guo*, Ping Chen*, Zhenfeng Xi*.Natl. Sci. Rev. 2022. DOI: 10.1093/nsr/nwac168. (#Authors contributed equally.)
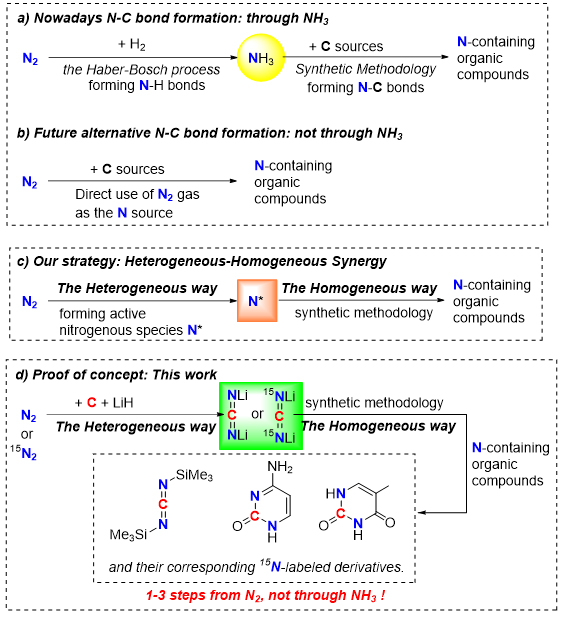
The element nitrogen and nitrogenous compounds are vital to life. The synthesis of nitrogen-containing compounds using dinitrogen as the nitrogen source, not through ammonia, is of great interest and great value but remains a grand challenge. Herein, we describe a strategy to realize this transformation by combining the heterogeneous approach with the homogeneous methodology. The N2 molecule was first fixed with carbon and LiH through a one-pot heterogeneous process, forming Li2CN2 as an ‘activated’ nitrogen source with high efficiency. Then subsequent homogeneous treatments of Li2CN2 to construct the organic synthon carbodiimide and the RNA/DNA building block pyrimidines were fulfilled. By using 15N2 as the feedstock, their corresponding 15N-labeled carbodiimide and pyrimidines were readily obtained. This homogeneous-heterogeneous synergy strategy will open a new chapter for N2 transformation.