研究室工作进展 Jun. 15th, 2015
Semibullvalene and Diazasemibullvalene: Recent Advances in the Synthesis, Reaction Chemistry, and Synthetic Applications
Shaoguang Zhang, Wen-Xiong Zhang, and Zhenfeng Xi*
Acc. Chem. Res. 2015, DOI:10.1021/acs.accounts.5b00190
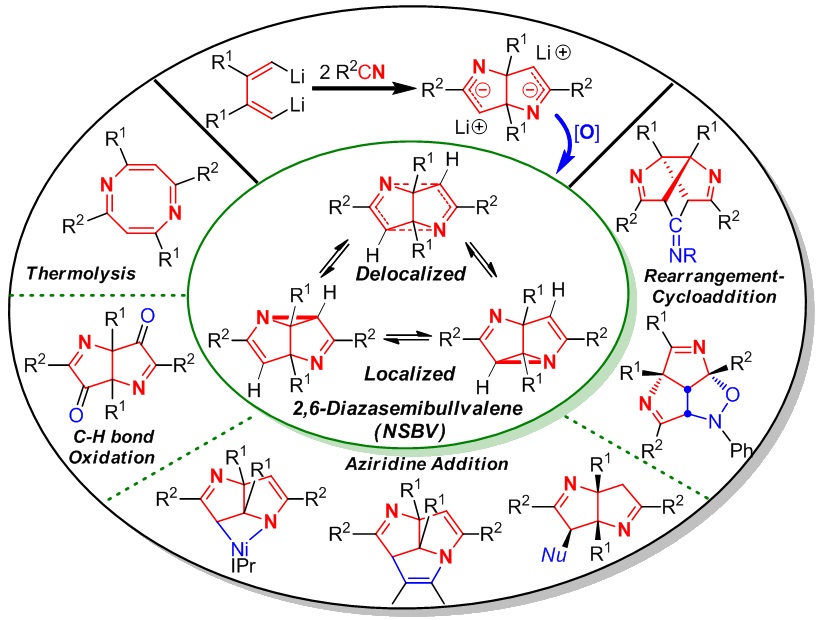
Semibullvalene (SBV) and its aza-analogue 2,6-diazasemibullvalene (NSBV) are both theoretically interesting and experimentally challenging organic molecules because of four unique features: highly strained ring systems, intramolecular skeletal rearrangement, extremely rapid degenerate (aza)Cope rearrangement, and predicted existence of neutral homoaromatic delocalized structures. SBV has received much attention in the past 50 years. In contrast, since NSBV was predicated in 1971 and the first in situ synthesis was realized in 1982, no progress on NSBV chemistry was made until our results in 2012. We have been interested in reaction chemistry of 1,4-dilithio-1,3-butadienes (dilithio reagents for short), especially for its application in synthesis of SBV and NSBV. This is because: i) the cyclodimerization of dilithio reagents could provide the potential 8-carbon skeleton of SBV from butadiene 4-carbon unit, and ii) the insertion reaction of dilithio reagents with C≡N bonds of two nitriles could provide the (6-C+2-N) skeleton which might be a good precursor for the synthesis of NSBV. Therefore, we initiated a journey to the synthesis and reaction chemistry of SBV and NSBV starting from dilithio reagents since 2006. In this Account, we outline mainly our recent achievement on the synthesis, structural characterization, reaction chemistry, synthetic application, and theoretical/computational analysis of NSBV.
Two efficient synthetic strategies for NSBV from dilithio reagents and nitriles via oxidant-induced C-N bond formation will be introduced. The structural investigation of NSBV, including X-ray crystal structure, determination of the activation barrier of aza-Cope rearrangement, and theoretical analysis, shows that the localized structure of NSBV is the predominant form, and the homoaromatic, delocalized structure exists as a minor component in the equilibrium. Then we will introduce the reaction chemistry and synthetic applications of NSBV. Several novel reaction patterns were explored including thermolysis, C-N bond insertion, rearrangement-cycloaddition, oxidation and nucleophilic ring-opening reaction. Diverse and interesting N-containing polycyclic skeletons could be constructed, such as nickelaazetidine, 1,5-diazatriquinacenes and triazabrexadienes, which are not available by other means.
Our results show that NSBV not only features rapid (aza)Cope rearrangement with low activation barrier, but also acts as unique synthetic reagent, which is significantly different from aziridine. The strained rigid ring systems as a whole could be involved in the reactions. Our achievement highlights two significant progresses: i) The well-established efficient synthesis and isolation of NSBV greatly accelerate the development of NSBV chemistry, and ii) The previously unattainable molecules have become “normal” and routine starting materials for the synthesis of otherwise unavailable but interesting structures. We expect our pursuits will inspire and help direct future efforts on chemical and physical research of NSBV.
亮点介绍
半瞬烯(Semibullvalene)和氮杂半瞬烯(Diazasemibullvalene)具有稠合的多个高张力环骨架、可以发生快速科普(Cope)重排、理论预测最有可能实现中性同芳香性(homoaromaticity),因此,在2000年前的近半个世纪中曾经引起物理有机化学家和有机合成实验化学家的极大关注,是有机化学中的“明星”分子,在有机化学中具有特殊意义。但是,与半瞬烯的活跃研究报道相比,氮杂半瞬烯的合成方法、结构、反应与应用知之甚少,在本组的近期工作报道之前几乎一无所知。
2006年本组王超同学利用本组自己发展的双锂试剂(注:本组发展的3个双锂试剂已经被J&K百灵威科技公司商品化并上市销售),在CuCl的促进下发生分子间自由基环化二聚,首次实现了金属促进的多取代半瞬烯的合成(JACS 2006)。该工作使我们有机会了解了半瞬烯和氮杂半瞬烯的研究历史、科学意义和研究现状。在此基础上,我们开展了更有挑战性的氮杂半瞬烯的合成研究。经过多位同学历经5年多的探索,最终于2012年由张韶光同学再次利用本组发展的双锂试剂的协同效应,建立了高效合成该类分子的2种方法,首次测定了该类分子的活化能和单晶结构,发现了该类分子的多种化学反应类型。该项研究结果表明该类分子存在中性同芳香性,为进一步的实验研究奠定了基础(JACS 2012,该文被选为JACS Spotlights)。进一步地,张韶光等同学开展了该类化合物的结构和反应性关系研究,发现了新的反应类型,利用该类化合物多个高张力环骨架的协同效应,“牵一发而动全身”,实现了一些具有特殊结构如碗状或空腔分子的合成(ACIE 2013, CC 2013, CEJ 2014, OCF 2014, CAJ 2015, OL 2015)。
该创新性、系统性工作建立在本组双金属有机合成试剂化学(ACR 2010) 的基础之上,是本组一直秉承的“基于活性中间体和机理研究的合成化学”研究方法(W.-X. Zhang and Z. Xi, Org. Chem. Front. 2014, 1, 1132)的成果之一。
该文是本组第三次在ACR期刊上介绍本组创新性和系统性工作。2010年ACR期刊介绍了本组的双金属有机合成试剂化学,2011年ACR期刊介绍了本组的金属有机杂环试剂化学。
