研究室工作进展 May. 16th, 2014
Diastereoselective Nucleophilic Ring-Opening Reactions of 2,6-Diazasemibullvalenes for the Synthesis of Diverse Functionalized Δ1-Bipyrroline Derivatives
Shaoguang Zhang, Ming Zhan, Wen-Xiong Zhang, and Zhenfeng Xi*
Chem. Eur. J. 2014, DOI: 10.1002/chem.201402911
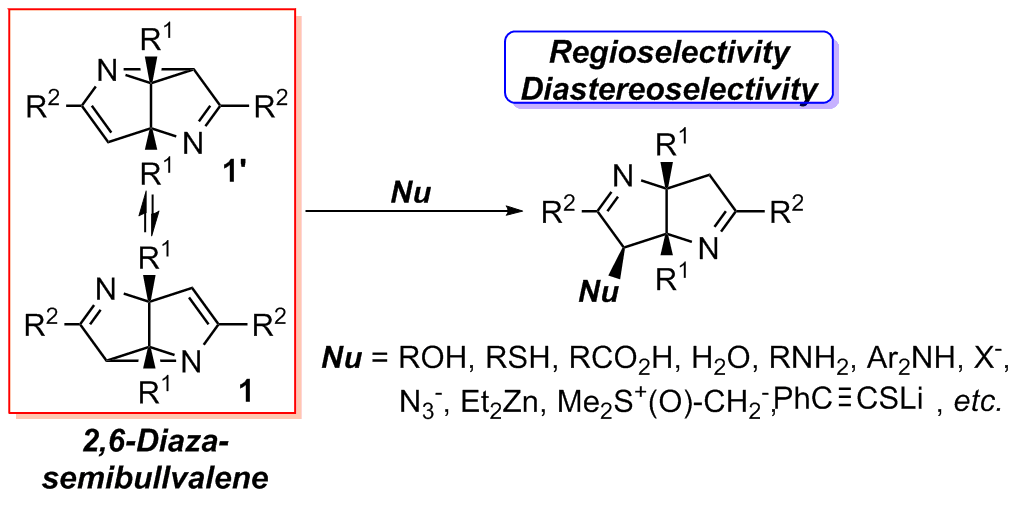
2,6-Diazasemibullvalenes are a class of structurally and chemically interesting polycyclic strained compounds. However, their reaction chemistry and synthetic applications are rarely explored.
Nucleophilic ring-opening reactions of 2,6-diazasemibullvalenes (NSBVs) were investigated. Different types of nucleophiles including alcohol, phenol, thiol, carboxylic acid, water, enol, amine, indole, metal halide salts, sodium azide, organozinc compounds, lithium alkynyl thiolates and sulfoxonium ylides were used, affording diverse functionalized Δ1-bipyrroline derivatives in good yields with high regio- and diastereo-selectivity. Most of the reactions featured milder conditions and higher reactivity compared with those for common aziridine derivatives, probably owing to the rigid ring system and substitution patterns of NSBVs.
This study not only provides an efficient synthetic method for various Δ1-bipyrroline derivatives, but also adds valuable knowledge to the understanding of the reaction chemistry of diazasemibullvalenes, a class of unique compounds.
亮点介绍
氮杂半瞬烯 (NSBV)是一类具有特殊骨架和性质的有机分子,曾经得到理论物理有机化学家的很大关注。本实验室利用自己发展的双锂试剂,于2012年成功高效地合成了该类化合物。之后,我们对该类化合物的反应化学和应用展开了研究。本工作结果再次说明该类化合物的新颖反应性和在合成一些特殊结构有机分子方面的有用性。本课题已经发表的论文如下。
张韶光、魏俊年、湛明等,J. Am. Chem. Soc. 2012, 134, 11964; Angew. Chem. Int. Ed. 2013, 52, 3485; Chem. Commun. 2013, 49, 6146.
