研究室工作进展 Jan. 25th, 2013.
Synthesis, Characterization, and Reactivity of N-Acyl Chloroformamidines: Useful Building Blocks for Construction of N-Acyl Ureas, Thioureas, Amidines and 1,1-Diaminoethylene
Yang Wang, Yue Chi, Fei Zhao, Wen-Xiong Zhang,* and Zhenfeng Xi
Synthesis 2013, 45, 347-354.
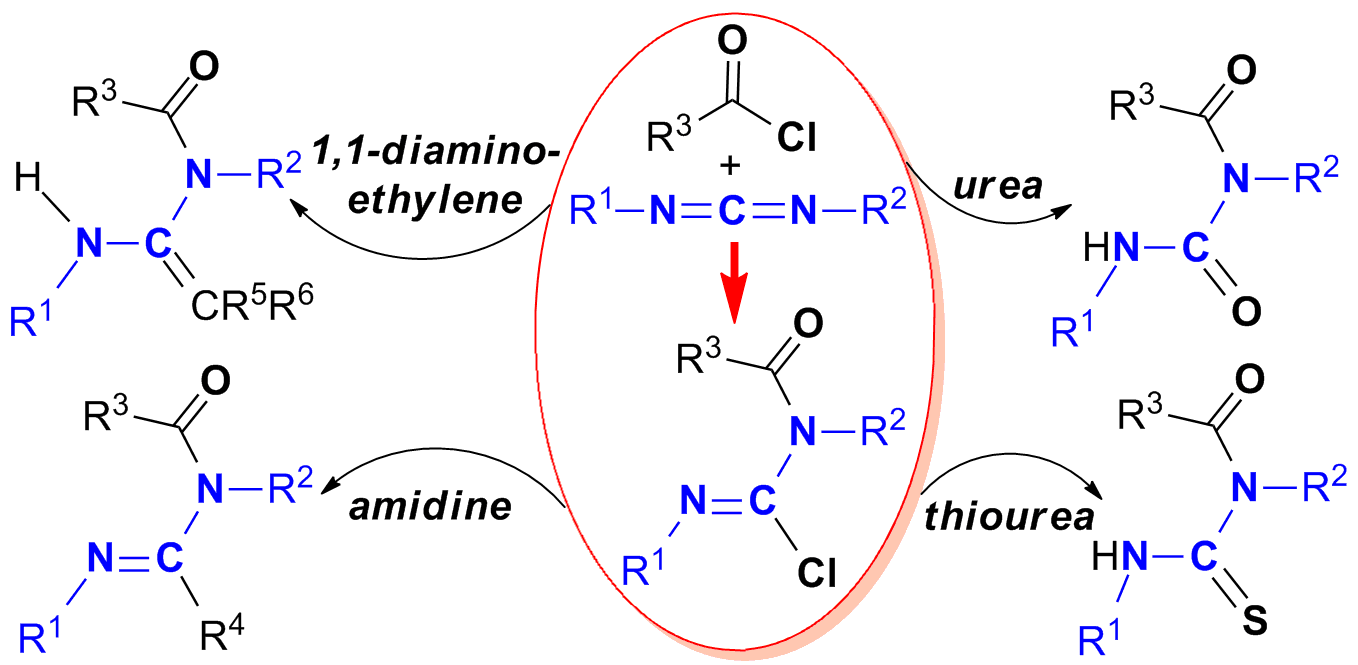
The systematic synthesis of N-acyl chloroformamidines are achieved by reaction of carbodiimides with acid chlorides, one of whose structures is assured by X-ray diffraction. Further reactivities of N-acyl chloroformamidines towards diethyl malonate, phenylethynyllithium, pentane-2,4-dione, methyl acetoacetate, water, 1,3-dimethyl(thio)urea and sodium hydrosulfide, provide unique protocols for the synthesis of N-acyl 1,1-diaminoethylene, amidine, urea and thiourea, respectively. These results show that N-acyl chloroformamidines have good ability to accept nucleophilic attack to construct useful N-containing compounds.
