研究室工作进展 Jan. 9th, 2017
Formation of Cyclopenta[c]pyridine Derivatives from 2,5-Disubstituted Pyrroles
and 1,4-Dibromo-1,3-butadienes via Pyrrole-Ring One-Carbon Expansion
Jianhao Yin, Qingyu Ye, Wei Hao, Shuaijing Du, Yucheng Gu, Wen-Xiong Zhang,* and Zhenfeng Xi*
Org. Lett. 2017, 19, 138-141.
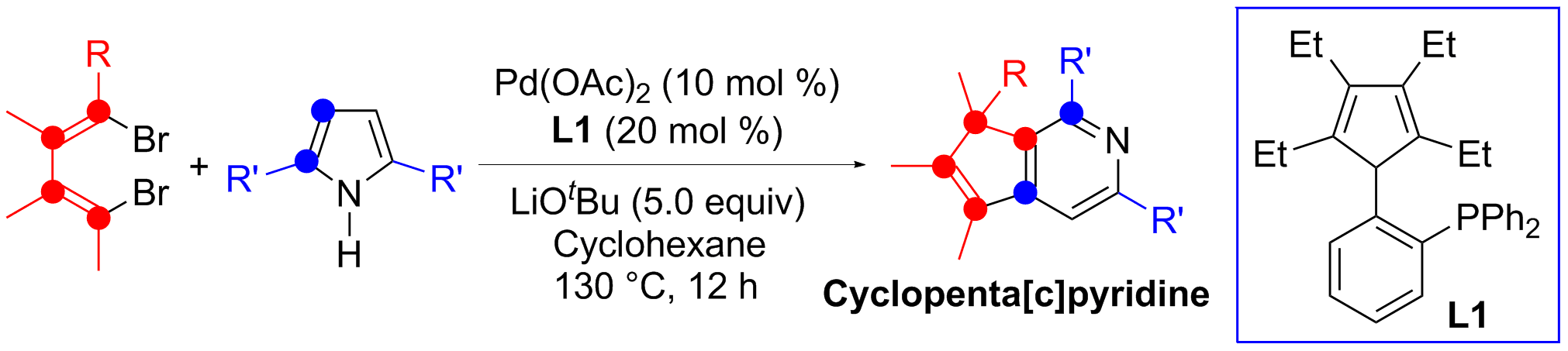
Ring-expansion reaction is a useful strategy in organic chemistry for the synthesis of larger cyclic compounds. Both five-membered pyrrole derivatives and six-membered pyridine derivatives are common compounds and useful building blocks. One-carbon expansion of the pyrrole skeleton should lead to the formation of pyridine derivatives. However, although it has been known for a long time that pyrrole can be transformed to pyridine with a dihalomethane and a strong base (the Reimer-Tiemann reaction conditions), such a transformation via one-carbon expansion of the pyrrole skeleton forming pyridine derivatives has not been well developed.
In this work, reactions between 1,4-dibromo-1,3-butadienes and 2,5-disubstituted pyrroles afforded cyclopenta[c]pyridine derivatives in high-yield, catalyzed by palladium and cyclopentadiene-phosphine ligand (L1) developed in our lab. Insertion of one terminal carbon of the butadienyl skeleton into one C=C double bond in the pyrrole ring resulted in ring expansion, along with 1,2-shift of an alkyl or an aryl substituent on the butadienes.
Note: This paper was introduced on “Follow ACS: Twitter Accounts”.
