研究室工作进展 Feb. 28th, 2018
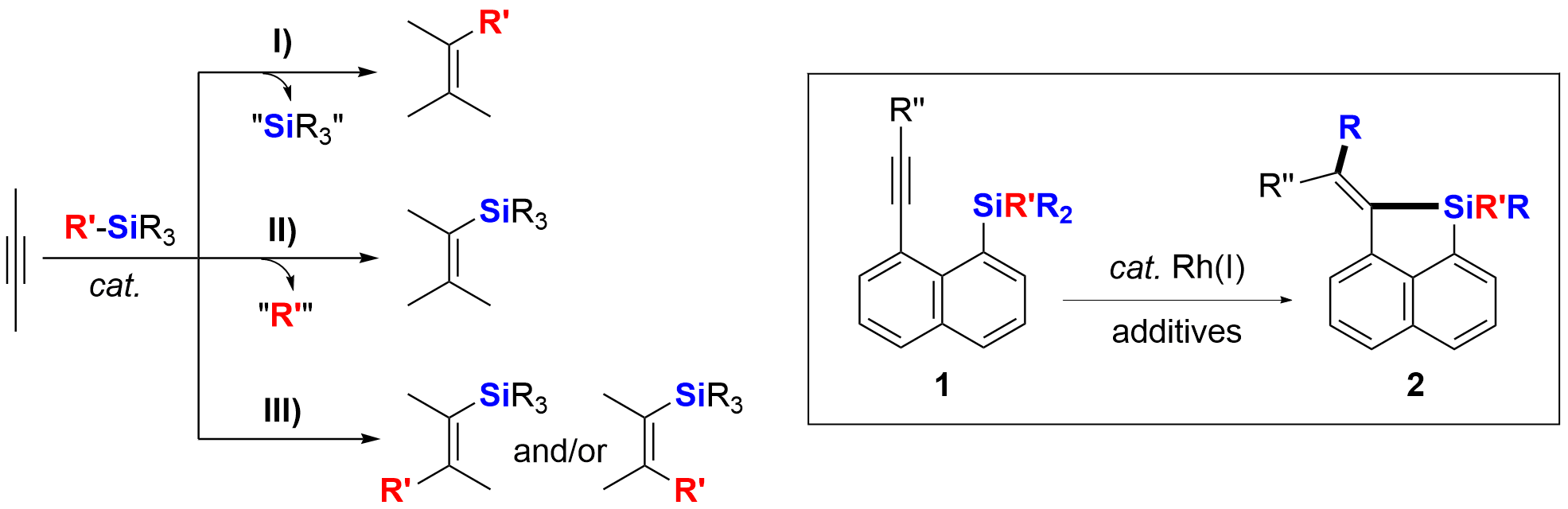
Selective cleavage of C(sp3)-Si bonds and their further synthetic applications catalyzed by transition-metal complexes or Lewis acids have attracted much recent attention. The scheme summarizes and illustrates three types of reactions between an alkyl-Si moiety and an alkynyl moiety via cleavage of C(sp3)-Si bonds. The alkyl-Si moiety and the alkynyl moiety can be either in two different molecules (eg. intermolecular reaction pattern) or in the same molecule (eg. intramolecular reaction pattern). For the case of I), an alkyl-Si moiety behaved as an alkylation reagent forming a new C(sp3)-C bond. The Si atom/moiety is released and is not incorporated into the targeted products. Compared with the reaction type I), there are more examples of II) in the literature. In this reaction protocol, cleavage of the alkyl-Si bond is followed by the formation of a new Si-C bond, along with release of the cleaved R′ moiety. Intramolecular reaction pattern of II) has resulted in a series of silole-containing compounds, which are very important for π-conjugated materials science. On the contrary, however, few examples of the reaction type III) are known in the literature.
As our continued interest in the synthesis of silacycles via cleavage of C-Si bonds, we found that 1-trimethylsilyl-8-alkynyl naphthalene derivatives 1 [SiR’R2 = SiMe3 (1a), SiEt3 (1b), Si(i-Pr)Me2 (1c), SiPhMe2 (1d)] could undergo a Rh(I)-catalyzed cis-selective carbo-silylation reaction to afford silacyclic compounds 2 in good yields. The Me-Si bond in 1a and the Et-Si bond in 1b could be efficiently cleaved, while the Me-Si bond in 1c, 1d and 1e, other than the i-Pr-Si bond in 1c and the Ph-Si bond in 1d and 1e, was preferentially cleaved.
