Research, news, May. 17th, 2013.
Mechanistic Study on the Cleavage and Reorganization of C(sp3)-H and C=N Bonds in Carbodiimides: Synthesis of 1,2-Dihydrothiopyrimidines and 2,3-Dihydropyrimidinthiones via Four-Component Coupling
Yang Wang, Fei Zhao, Yi Zhou, Yue Chi, Zitao Wang, Wen-Xiong Zhang,* and Zhenfeng Xi
Chem. Eur. J. 2013, DOI: 10.1002/chem.201301633.
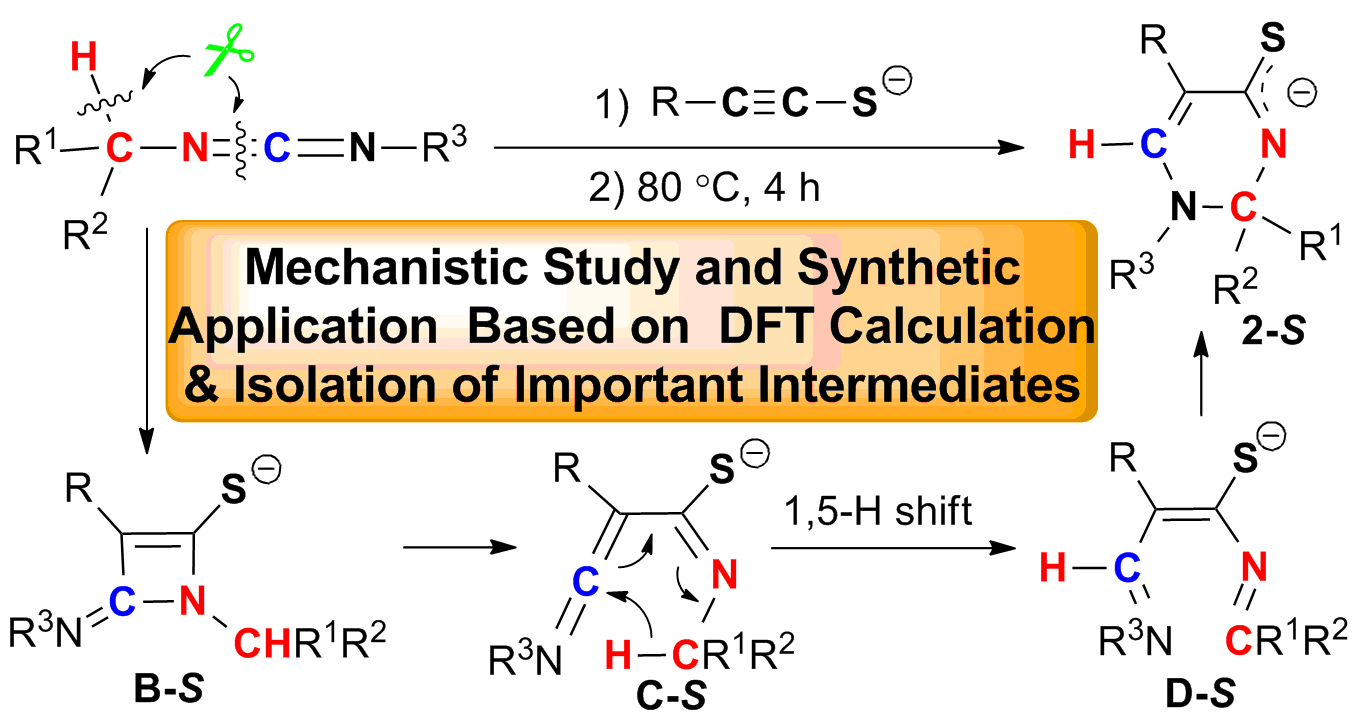
This study reveals the light of dawn on the cleavage and reorganization of C(sp3)-H and C=N bonds of carbodiimides in this three-component reaction of terminal alkynes, sulfur, and carbodiimides by the combined methods including (i) isolation and X-ray structure of six-membered-ring lithium species 2-S, (ii) trapping of the Oxygen-analogues (B-O and D-O) of both four-membered-ring intermediate B-S and ring-opening intermediate D-S, (iii) deuterium labeling studies, (iv) theoretical study. These results show that (i) this reaction rate-determining step is [2+2] cycloaddition, (ii) the C=N bond cleavage takes place before the C(sp3)-H bond cleavage, (iii) the hydrogen attached to the C6 in 2-S is from carbodiimide, (iv) three types of new aza-heterocycles, such as 1,2-dihydrothiopyrimidines, N-acyl 2,3-dihydropyrimidinthiones and 1,2-dihydropyrimidinamino acids are constructed efficiently based on 2-S. All results strongly support that the reaction undergoes through [2+2] cycloaddition/4π electrocyclic ring-opening/1,5-H shift/6π electrocyclic ring-closing as key steps. The research strategy on the synthesis, isolation and reactivity investigation of important intermediates in metal-mediated reactions not only gives the in-depth understanding of reaction mechanisms but also leads to the discovery of new synthetically useful reactions based on the important intermediates.
