Research News, Nov 1, 2009
研究室工作进展 Nov. 1, 2009
One-pot Multicomponent Synthesis of Azaindoles and Pyrroles from One
Molecule of a Silicon-Tethered Diyne and Three or Two Molecules of
Organonitriles Mediated by Zirconocene
Shaoguang Zhang, Xiaohua Sun, Wen-Xiong Zhang, and Zhenfeng Xi*
Chem. Eur. J. 2009, ASAP online October 31
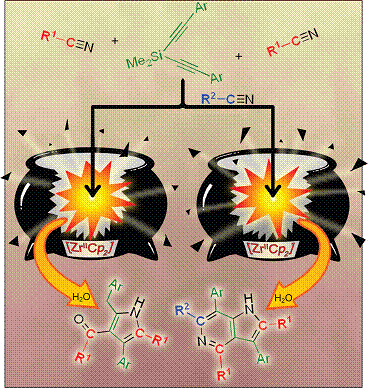
A zirconocene-mediated one-pot multi-component synthesis process has been developed, leading to the synthesis of 5-azaindoles and pyrrole derivatives, from a Si-tethered diyne and three or two different or identical organonitriles. 5-Azaindoles and pyrrole derivatives of diversified structures and substitution patterns could be highly selectively prepared via this protocol. A wide variety of organonitriles and Si-tethered diynes, either aliphatic or aromatic with both electron-withdrawing groups and electron-donating groups, could be used to afford 5-azaindoles and/or pyrroles in good-to-excellent isolated yields. A key intermediate formed in the Cp2Zr(II)-mediated reaction of one (PhC≡C)2SiMe2, two molecules of i-PrCN and one p-TolylCN was characterized by X-ray single-crystal structural analysis, which has allowed us to gain a good understanding of the reaction process.
