Research News, Feb 23, 2011
研究室工作进展Feburary23, 2011
Silyl-substituted 1,3-butadienes for Diels–Alder reaction,
ene reaction and allylation reaction
Fei Zhao, Shaoguang Zhang, and Zhenfeng Xi*
Chem. Commun. 2011, doi: 10.1039/c0cc05665k (Feature Article)
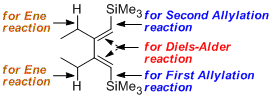
The introduction of SiMe3 groups into 1,3-butadiene skeleton establishes several new reaction sites: the 1,3-diene moiety for Diels-Alder [4+2] cycloaddition, the alkyl-substituted olefin moiety for ene reaction, and the SiMe3-substituted olefin for allylation and desilylation. The cooperation between the silyl group and the conjugated diene skeleton makes silyl-substituted 1,3-butadienes very unique.This Feature Article reviews the synthesis of silyl-substituted 1,3-butadienes, and their applications in Diels-Alder reaction, ene reaction and allylation. Most notably, silyl-substituted 1,3-butadienes readily participate in different tandem reactions such as Diels-Alder/allylation, ene/allylation, ene/allylation/Diels-Alder reaction, ene/allylation/ene reaction and ene/allylation/Diels-Alder/allylation reaction.
