Owing to the multi-stimuli responsiveness and robust dynamic exchange properties of disulfide bonds, poly(disulfide)s have attracted growing attention in many areas, such as biomedical and energy materials, gels and elastomers, recyclable or reprocessable materials, and even the creation of new materials such as elastic ceramics, representing a class of cutting-edge polymeric materials with a broad range of applications. The conventional synthesis of poly(disulfide)s mainly relies on polycondensation reaction, which is difficult to control. In contrast, the ring-opening polymerization of cyclic disulfide, such as the commercially available lipoic acid and its derivatives, has gradually become an important method for preparing functional poly(disulfide)s. However, due to the high reactivity of the sulfide chain ends, there are two main challenges faced in the synthesis of poly(disulfide)s (Fig. 1): 1) Significant chain transfer reaction, leading to poor control over polymerization and broad molecular weight distribution; 2) The low regioselectivity in the ring-opening reaction of lipoate monomers, resulting in polymers with low regioregularity and thermal properties.
Recently, Yun Liu and his research group at Peking University developed an "anion-binding catalysis" strategy to achieve controlled ring-opening polymerization of lipoic acid derivatives with high regioselectivity via a thiourea-tetramethylguanidine catalytic system. The obtained poly(disulfide)s feature low dispersity (1.1–1.2) and high regioselectivity (head-to-tail ratio > 85%) (Fig. 1).
伟德国际victor·1946(CHINA)官方网站 -登录入口
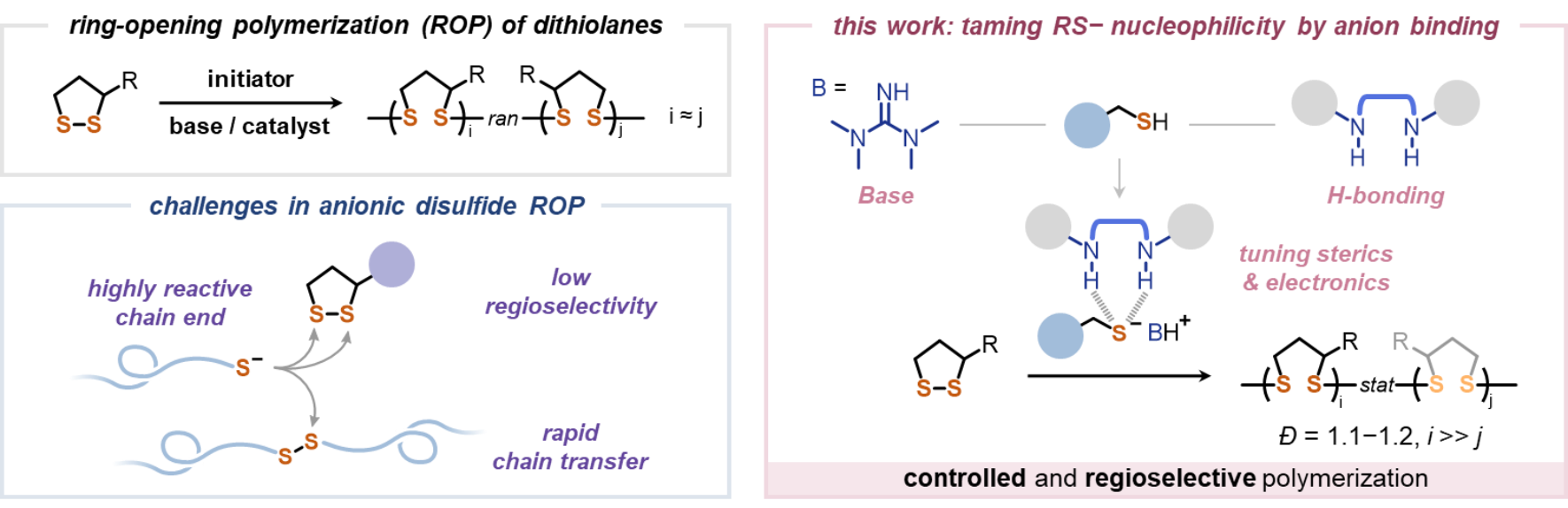
Fig. 1 Synthesis of poly(disulfide): challenges and solutions.
They found that tetramethylguanidine partially deprotonates the thiol initiator so that the protons serve as a reversible chain termination reagent to establish an improved control over polymerization. The introduction of an epiquinine-based thiourea catalyst which contains a tertiary amine (Fig. 2) accelerated the rate of polymerization while maintaining the control. Kinetic experiments showed that the rate of propagation is three times faster than those of other catalysts. Furthermore, the ring-opening polymerization exhibits the characteristics of a living polymerization process: linear relationships are found between the molecular weight of the polymer and monomer conversion or monomer-initiator ratio.
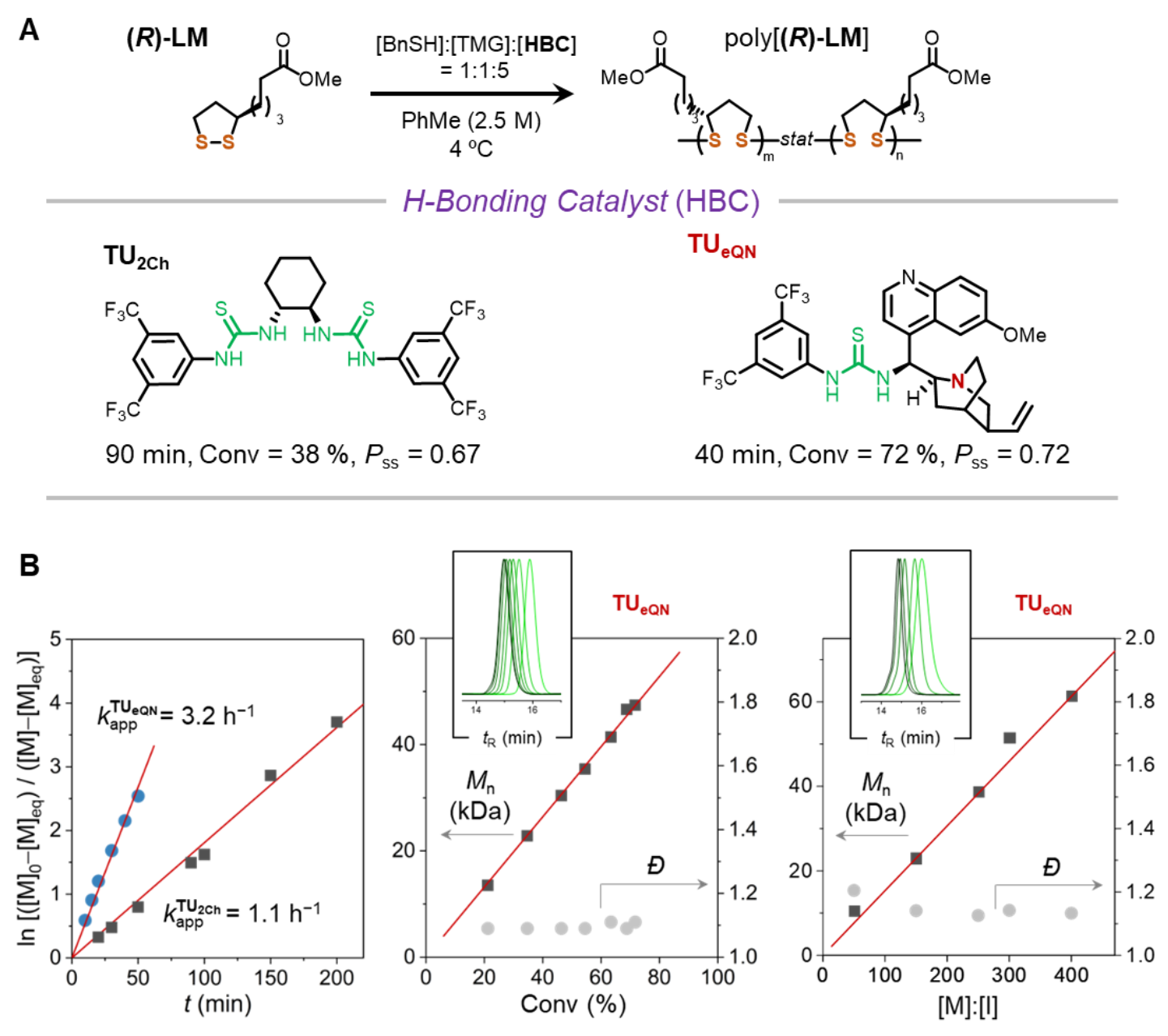
Fig. 2 Thiourea catalyst achieves poly(disulfide) with rapid kinetics, low dispersity, and high regioselectivity (Pss).
The living polymerization conditions are amenable to monomers with various functional groups. Non-polar, polar, fluorous and olefin-containing monomers were successfully polymerized with narrow dispersity and high regioregularity. Through the adjustment of regioregularity, the authors found that the lipoate-based polymers with long side chains gradually become semi-crystalline, despite they have previously been considered to be amorphous. The enthalpy of melting shows positive correlation with the regioregularity (Fig. 3), an often underappreciated aspect in engineering the physical properties of polymers.
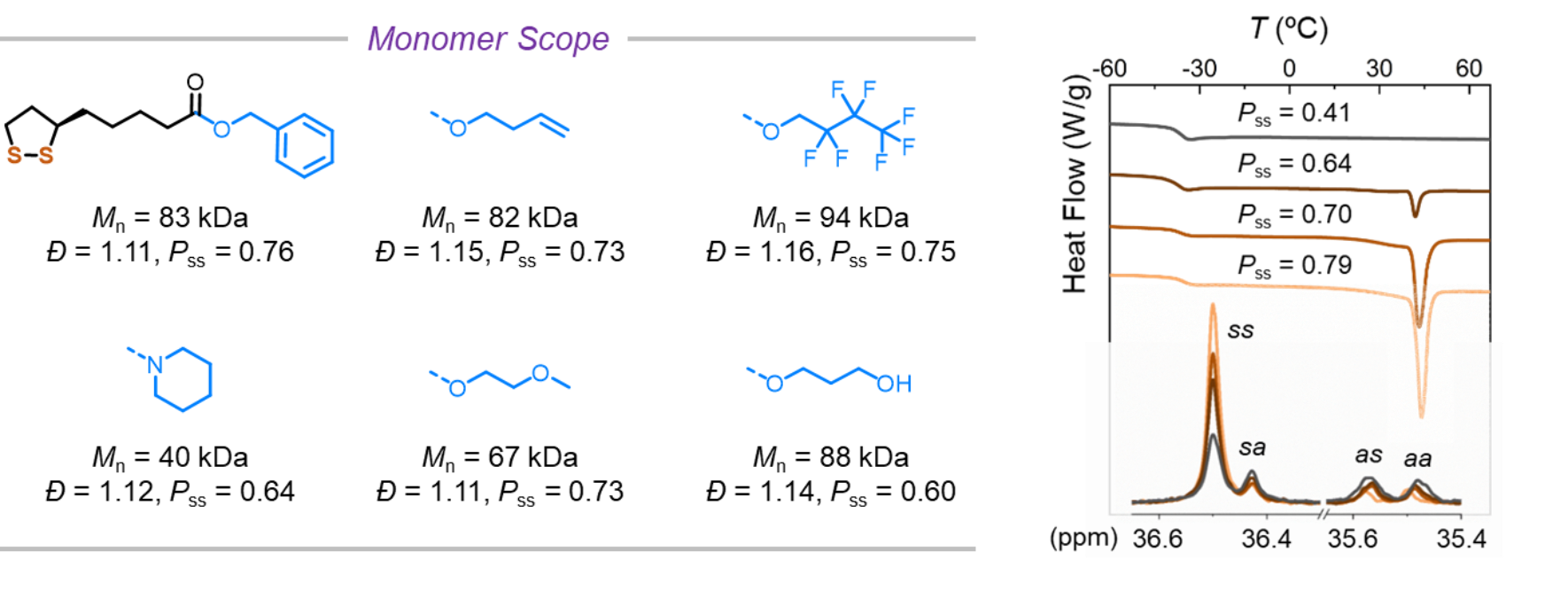
Fig. 3 Monomer scope (left); The crystallization peak of poly(R-methyl lipoate) at 45 ºC increases with the increasing regioregularity (Pss) (right).
In general, reactivity and selectivity are mutually exclusive: Improving reactivity usually means sacrificing reaction selectivity. Based on exhaustive experimental studies of polymerization mechanisms and density functional theory calculations, the authors revealed that a catalyst-guanidinium-sulfide ternary complex is the active species in propagation. Within this complex, the catalyst and the base together form a spatially congested monomer insertion pocket, which improves the regioselectivity of the ring-opening step. At the same time, guanidinium stabilizes the forming chain end anion at the transition state of ring-opening reaction through electrostatic interactions and hydrogen bonding, accelerating the ring-opening step while promoting the reaction selectivity. This unusual ground state-transition state dual stabilization mode is complementary to the known hydrogen bond catalysis modes, i.e., LUMO-lowering or anion activation mode. This new mode deepens our understanding of hydrogen bonding catalysis with great potential to be applied in other ring-opening polymerizations.
This work has been recently published in the Journal of the American Chemical Society, titled "Controlled and Regioselective Ring-Opening Polymerization for Poly(disulfide)s by Anion-Binding Catalysis". Yun Liu, an assistant professor at the College of Chemistry and Molecular Engineering of Peking University, and Peiyuan Yu, an assistant professor at the Department of Chemistry of South University of Science and Technology are corresponding authors. The first author is Tianyi Du, a Ph.D. student from Liu group, and the co-first author is Boming Shen from Yu’s group. The work is supported by the Beijing National Laboratory for Molecular Science and National Natural Science Foundation of China.
Link to the paper: https://pubs.acs.org/doi/full/10.1021/jacs.3c10708.