Polystyrene is one of the most important materials in the modern plastics industry and is widely used in electronics, food containers, packaging and building materials. Polystyrene is also well suited as a raw material for obtaining high-value materials with new properties and extended utility through functionalization. However, due to the low reactivity of aromatic hydrocarbon bonds in polystyrene, its directfunctionalizationremains a considerable challenge. In the past, the methods studied included Friedel−Crafts alkylation and acylation, halomethylation,perfluoroalkylation, sulfonation, nitration, and alkyl Lithium metalation or super base-metalation. However, these methods often suffer from problems such as harsh reaction conditions and chain degradation and cross-linking, resulting in inefficient and uncontrollable functionalization. Among the various methods of polystyrene functionalization, the halogenation of aromatic C―H bonds is very attractive because the halogenated products can be utilized for the subsequent transformation to access a series of functionalized polystyrenes. Although the halogenation of polystyrene has been studied for a long time, most of the protocols developed so far are also accompanied by the problems such as chain degradation and cross-linking. Besides, the degree of halogenation cannot be precisely controlled.
Recently, the research team of Jianbo Wang from the College of Chemistry and Molecular Engineering of Peking University has developed a highly efficient gold-catalyzed halogenation of polystyrene (J. Am. Chem. Soc. 2023, 145, 10422-10430). This halogenation method proceeds highly selectively at the para-position of the polystyrene benzene ring, while maintaining the polystyrene chain structure without any degradation and cross-linking. The more important feature of this method is that the degree of halogenation can be precisely controlled by simply changing the loading of halogenating agent, allowing the density of functional groups to be adjusted in an accurate and predictable manner (Fig. 1).
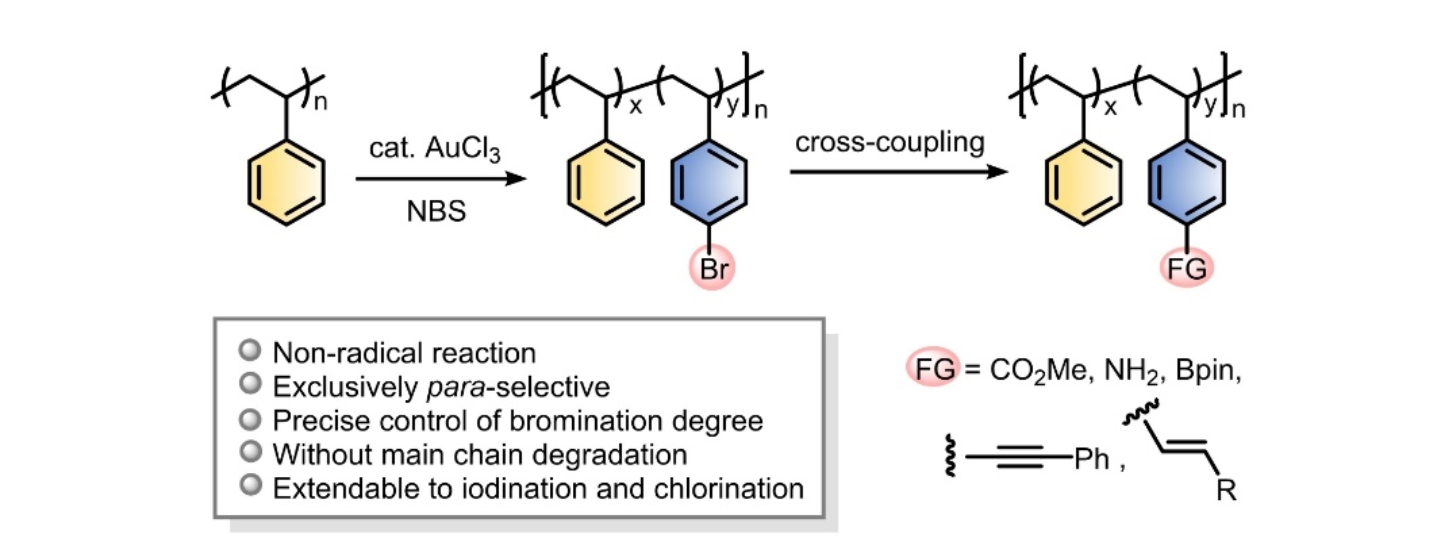
Fig.1 Gold(III) chloride-catalyzed halogenation and functionalization of polystyrene.
This controllable halogenation method is based on the gold(III) chloride-catalyzed halogenation developedby Jianbo Wang's research teamin2010(Angew. Chem. Int. Ed. 2010, 49, 2028-2032). Due to its high efficiency and very mild reaction conditions, this reaction has been used in drug discovery, functional material synthesis, as well as complex natural product synthesis. In the application of this reaction in the halogenation of polystyrene, they first adjusted and optimized the bromination conditions and found thatwith 1 mol% AuCl3 as the catalyst, stoichiometric N-bromosuccinimide (NBS) as the bromination reagent, almost quantitative bromination of polystyrenecould be realizedwithout affecting the integrity of the polymerbackbone. Precise regulation of the degree of bromination was then achieved by simply changing the loading of NBS. This strategy could be extended to iodination and chlorination processes, however, the chlorination efficiency was slightly reduced. Through in-depth analytical characterization of the structure of the polymer, they confirmed that the halogenation process had a high degree ofpara-selectivity.
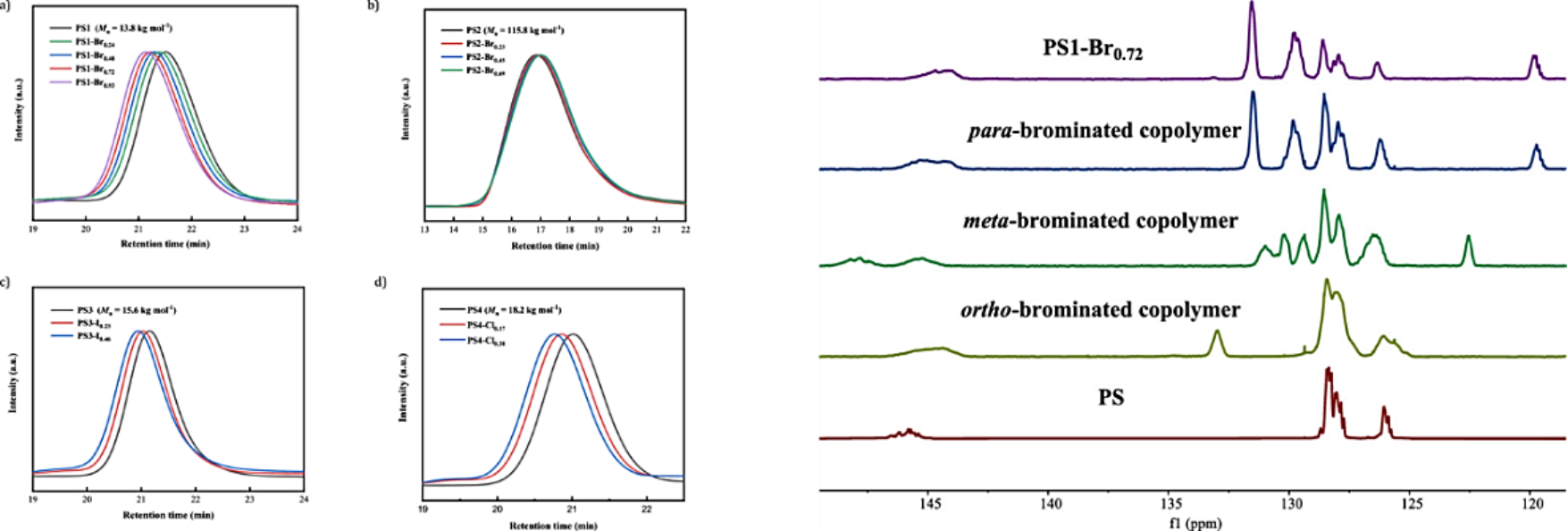
Fig.2 Confirmation of the controlled bromination of polystyrene: polymer chain integrity (left) and para-selectivity (right).
This research also provides anewstrategy for the precise synthesis of a series of functionalized polystyrene materials. For example, usingbrominated polystyrene as the raw material, combined with Heck reaction, Suzuki-Miyaura coupling, Miyauraborylation and Sonogashira coupling, efficient introduction of various functional groups such as alkenyl, aryl, boron ester and alkyne could be realized. The results show that the molecular weight distribution of brominated polystyrene remains virtually unchanged during these transformation, indicating that the integrity of the polymer chain is maintained. In addition, due to the highly controllable nature of the halogenation reaction, two orthogonal functional groups can be successfully introduced into the polymer chain by sequential functionalization, which provides an effective tool for synthesizing polystyrene materials with novel structures and complex functions(Fig.3). At the same time, this strategy could also realize the regulation of the thermal properties and hydrophilic properties of the materials by changing the density of the introduced functional groups.
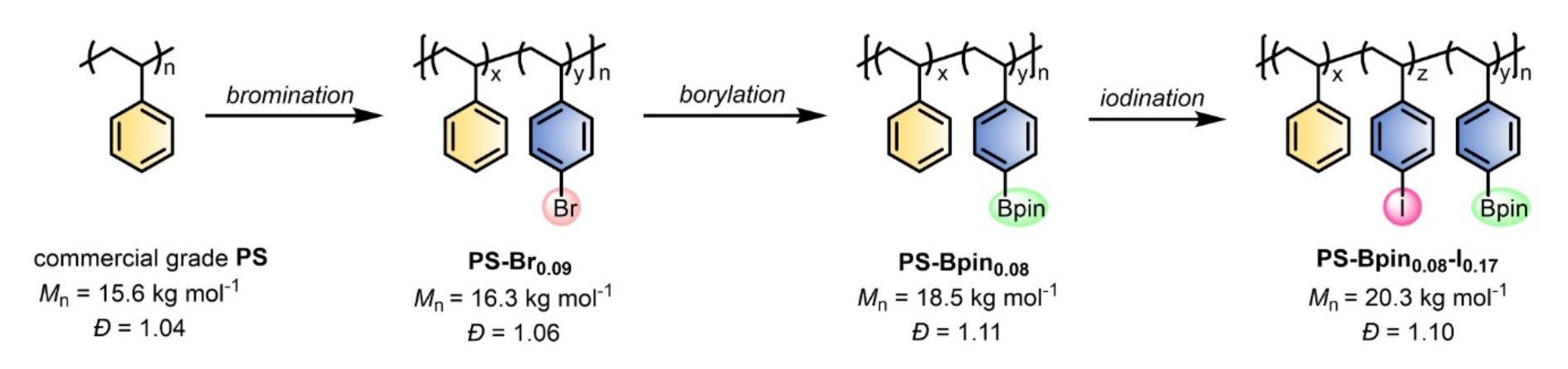
Fig.3 Successive controlled synthesis of bifunctional PS.
In addition to polystyrene, this method can be applied to a wide range of commercially available aromatic polymer materials. For example, syntactic polystyrene (sPS), substituted polystyrene, polystyrene copolymer and polyethylene terephthalate (PET)could be brominated by this method, further demonstrating the generality of this protocol. In conclusion, this researchprovides an efficient method for the modification of commercial polystyrenematerials.
Bowen Dou,a Ph.D.candidateat the College of Chemistry and Molecular Engineering of PekingUniversity is the first author of this paper. Prof.Jianbo Wang is the corresponding author, and Dr. Yan Xufrom the College of Chemistry and Molecular Engineering of Peking Universityis the co-author. This work is supported by the Beijing National Laboratory of Molecular Sciences.
Original link for the paper: https://pubs.acs.org/doi/full/10.1021/jacs.3c03069